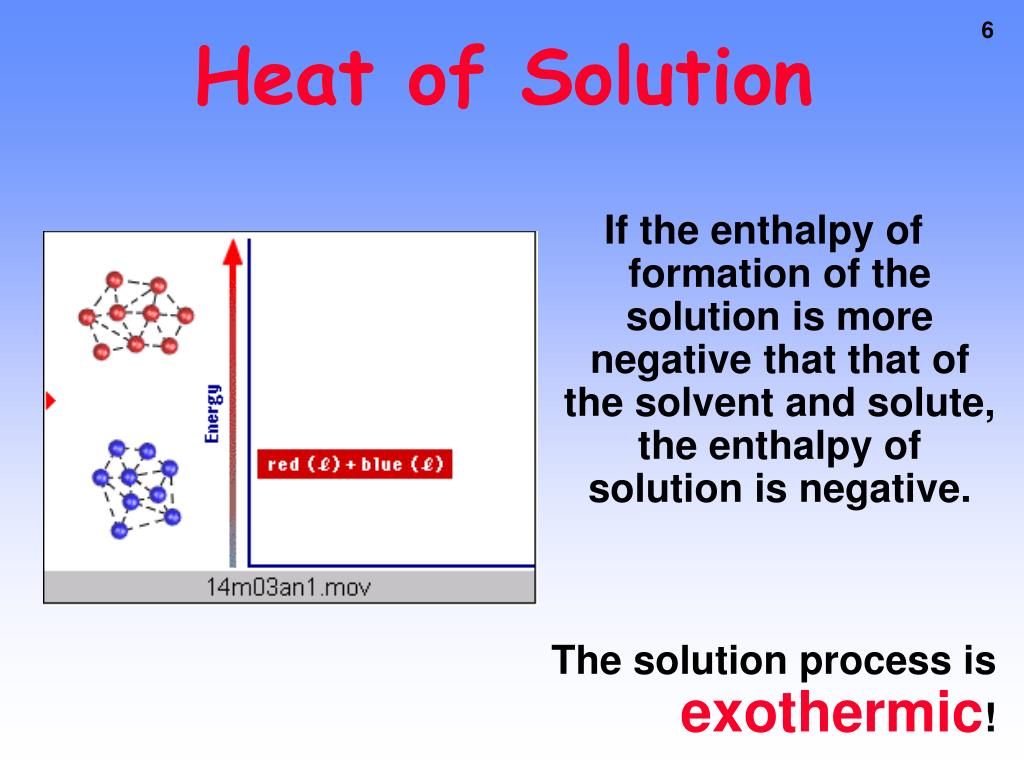
Standard Heat Of Solution . Web the molar heat of solution \(\left( \delta h_\text{soln} \right)\) of a substance is the heat absorbed or released when one mole of. The heat energy released when new bonds are made. Web to understand enthalpies of solution and be able to use them to calculate the heat absorbed or emitted when making solutions. Web the heat energy needed to break up 1 mole of the crystal lattice is the lattice dissociation enthalpy. The δhº rxn is the standard. Web enthalpies of solution 1. Web the heat of solution, also referred to the enthalpy of solution or enthalpy of dissolution, is the enthalpy change associated with the. The standard state of a solid or liquid is the pure substance at a pressure of 1 bar ( 10 5 pa) and at a relevant temperature.
from www.slideserve.com
Web enthalpies of solution 1. Web the molar heat of solution \(\left( \delta h_\text{soln} \right)\) of a substance is the heat absorbed or released when one mole of. The standard state of a solid or liquid is the pure substance at a pressure of 1 bar ( 10 5 pa) and at a relevant temperature. The heat energy released when new bonds are made. The δhº rxn is the standard. Web the heat energy needed to break up 1 mole of the crystal lattice is the lattice dissociation enthalpy. Web to understand enthalpies of solution and be able to use them to calculate the heat absorbed or emitted when making solutions. Web the heat of solution, also referred to the enthalpy of solution or enthalpy of dissolution, is the enthalpy change associated with the.
PPT EXOTHERMIC PowerPoint Presentation, free download ID5879266
Standard Heat Of Solution Web the heat energy needed to break up 1 mole of the crystal lattice is the lattice dissociation enthalpy. The heat energy released when new bonds are made. The δhº rxn is the standard. Web the heat of solution, also referred to the enthalpy of solution or enthalpy of dissolution, is the enthalpy change associated with the. Web enthalpies of solution 1. Web the heat energy needed to break up 1 mole of the crystal lattice is the lattice dissociation enthalpy. Web to understand enthalpies of solution and be able to use them to calculate the heat absorbed or emitted when making solutions. The standard state of a solid or liquid is the pure substance at a pressure of 1 bar ( 10 5 pa) and at a relevant temperature. Web the molar heat of solution \(\left( \delta h_\text{soln} \right)\) of a substance is the heat absorbed or released when one mole of.
From www.linstitute.net
CIE A Level Chemistry复习笔记5.1.7 Constructing Energy Cycles using Standard Heat Of Solution The heat energy released when new bonds are made. Web the heat of solution, also referred to the enthalpy of solution or enthalpy of dissolution, is the enthalpy change associated with the. Web enthalpies of solution 1. Web the heat energy needed to break up 1 mole of the crystal lattice is the lattice dissociation enthalpy. Web to understand enthalpies. Standard Heat Of Solution.
From ceuuptlx.blob.core.windows.net
Standard Enthalpy Of Formation To Zero at Deanne Fox blog Standard Heat Of Solution Web enthalpies of solution 1. Web the heat of solution, also referred to the enthalpy of solution or enthalpy of dissolution, is the enthalpy change associated with the. Web to understand enthalpies of solution and be able to use them to calculate the heat absorbed or emitted when making solutions. Web the heat energy needed to break up 1 mole. Standard Heat Of Solution.
From www.youtube.com
RELATIONSHIP BETWEEN HEAT OF SOLUTION,LATTICE AND HYDRATION ENERGY Standard Heat Of Solution The δhº rxn is the standard. Web the heat of solution, also referred to the enthalpy of solution or enthalpy of dissolution, is the enthalpy change associated with the. The heat energy released when new bonds are made. Web to understand enthalpies of solution and be able to use them to calculate the heat absorbed or emitted when making solutions.. Standard Heat Of Solution.
From childhealthpolicy.vumc.org
⚡ Heat of solution of kno3. What is the specific heat of kno3?. 20221026 Standard Heat Of Solution The standard state of a solid or liquid is the pure substance at a pressure of 1 bar ( 10 5 pa) and at a relevant temperature. Web the molar heat of solution \(\left( \delta h_\text{soln} \right)\) of a substance is the heat absorbed or released when one mole of. Web the heat energy needed to break up 1 mole. Standard Heat Of Solution.
From kunduz.com
[ANSWERED] 1 The standard heat of formation of sodium ions in aqueous Standard Heat Of Solution Web the heat energy needed to break up 1 mole of the crystal lattice is the lattice dissociation enthalpy. The standard state of a solid or liquid is the pure substance at a pressure of 1 bar ( 10 5 pa) and at a relevant temperature. Web to understand enthalpies of solution and be able to use them to calculate. Standard Heat Of Solution.
From www.youtube.com
specific heat and heat of solution lab YouTube Standard Heat Of Solution Web the heat energy needed to break up 1 mole of the crystal lattice is the lattice dissociation enthalpy. The heat energy released when new bonds are made. The δhº rxn is the standard. Web the molar heat of solution \(\left( \delta h_\text{soln} \right)\) of a substance is the heat absorbed or released when one mole of. Web the heat. Standard Heat Of Solution.
From materialelmore.z21.web.core.windows.net
Calculating Enthalpy Of Reaction Worksheet Standard Heat Of Solution Web to understand enthalpies of solution and be able to use them to calculate the heat absorbed or emitted when making solutions. Web enthalpies of solution 1. Web the heat energy needed to break up 1 mole of the crystal lattice is the lattice dissociation enthalpy. The δhº rxn is the standard. The heat energy released when new bonds are. Standard Heat Of Solution.
From studylib.net
Heat of Solution Standard Heat Of Solution Web the heat energy needed to break up 1 mole of the crystal lattice is the lattice dissociation enthalpy. Web enthalpies of solution 1. Web to understand enthalpies of solution and be able to use them to calculate the heat absorbed or emitted when making solutions. The δhº rxn is the standard. Web the heat of solution, also referred to. Standard Heat Of Solution.
From www.youtube.com
Heat of Reaction (from Heat of Formation) YouTube Standard Heat Of Solution Web enthalpies of solution 1. Web the molar heat of solution \(\left( \delta h_\text{soln} \right)\) of a substance is the heat absorbed or released when one mole of. The δhº rxn is the standard. The heat energy released when new bonds are made. Web the heat energy needed to break up 1 mole of the crystal lattice is the lattice. Standard Heat Of Solution.
From exoziqnph.blob.core.windows.net
Standard Heat Of Formation Co2 at Jonathan Blackburn blog Standard Heat Of Solution Web the heat of solution, also referred to the enthalpy of solution or enthalpy of dissolution, is the enthalpy change associated with the. Web to understand enthalpies of solution and be able to use them to calculate the heat absorbed or emitted when making solutions. The δhº rxn is the standard. The heat energy released when new bonds are made.. Standard Heat Of Solution.
From lessonlibrarytentwise.z14.web.core.windows.net
Heats Of Formation Formula Standard Heat Of Solution Web enthalpies of solution 1. Web to understand enthalpies of solution and be able to use them to calculate the heat absorbed or emitted when making solutions. Web the molar heat of solution \(\left( \delta h_\text{soln} \right)\) of a substance is the heat absorbed or released when one mole of. The standard state of a solid or liquid is the. Standard Heat Of Solution.
From pediaa.com
Difference Between Heat of Formation and Heat of Reaction Definition Standard Heat Of Solution Web the heat of solution, also referred to the enthalpy of solution or enthalpy of dissolution, is the enthalpy change associated with the. Web to understand enthalpies of solution and be able to use them to calculate the heat absorbed or emitted when making solutions. The heat energy released when new bonds are made. Web enthalpies of solution 1. The. Standard Heat Of Solution.
From www.slideserve.com
PPT EXOTHERMIC PowerPoint Presentation, free download ID5879266 Standard Heat Of Solution Web the molar heat of solution \(\left( \delta h_\text{soln} \right)\) of a substance is the heat absorbed or released when one mole of. Web to understand enthalpies of solution and be able to use them to calculate the heat absorbed or emitted when making solutions. The heat energy released when new bonds are made. The standard state of a solid. Standard Heat Of Solution.
From exokycsnc.blob.core.windows.net
Standard Enthalpy Of Formation Table Elements at Filomena Gilbert blog Standard Heat Of Solution Web the molar heat of solution \(\left( \delta h_\text{soln} \right)\) of a substance is the heat absorbed or released when one mole of. Web to understand enthalpies of solution and be able to use them to calculate the heat absorbed or emitted when making solutions. Web the heat of solution, also referred to the enthalpy of solution or enthalpy of. Standard Heat Of Solution.
From royalatila.weebly.com
Chemical reaction thermodynamics calculator royalatila Standard Heat Of Solution Web the heat of solution, also referred to the enthalpy of solution or enthalpy of dissolution, is the enthalpy change associated with the. The standard state of a solid or liquid is the pure substance at a pressure of 1 bar ( 10 5 pa) and at a relevant temperature. Web to understand enthalpies of solution and be able to. Standard Heat Of Solution.
From pdfprof.com
steps in preparing a standard solution Standard Heat Of Solution Web the heat energy needed to break up 1 mole of the crystal lattice is the lattice dissociation enthalpy. The δhº rxn is the standard. Web the heat of solution, also referred to the enthalpy of solution or enthalpy of dissolution, is the enthalpy change associated with the. The heat energy released when new bonds are made. Web enthalpies of. Standard Heat Of Solution.
From www.youtube.com
Enthalpy of Solution, Enthalpy of Hydration, Lattice Energy and Heat of Standard Heat Of Solution Web the heat energy needed to break up 1 mole of the crystal lattice is the lattice dissociation enthalpy. Web the molar heat of solution \(\left( \delta h_\text{soln} \right)\) of a substance is the heat absorbed or released when one mole of. The δhº rxn is the standard. The standard state of a solid or liquid is the pure substance. Standard Heat Of Solution.
From slideplayer.com
Chapter 16 Reaction Energy ppt download Standard Heat Of Solution The δhº rxn is the standard. The standard state of a solid or liquid is the pure substance at a pressure of 1 bar ( 10 5 pa) and at a relevant temperature. Web the molar heat of solution \(\left( \delta h_\text{soln} \right)\) of a substance is the heat absorbed or released when one mole of. Web the heat of. Standard Heat Of Solution.